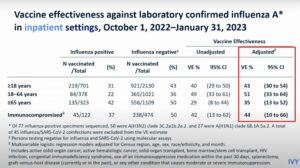
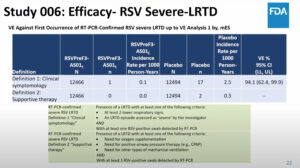
NEW YORK (Reuters Health) – In patients with psoriatic arthritis, once weekly etanercept (Enbrel) is suitable for joint symptoms but twice weekly dosing is needed to best address skin lesions, according to a report in the February 9th Online First issue of BMJ
“Inflammation in the skin seems to be more pronounced than in joints of psoriasis patients, and thus requires a higher dosing, particularly in the induction phase of treatment,” lead author Dr. Wolfram Sterry, from Charite University Medicine, Berlin, told Reuters Health by email.
According to Dr. Sterry, the trial was the first to compare “two different dosing regimens of etanercept in patients with psoriasis, suffering both from moderate to severe skin involvement as well as marked arthritis.”
The 98-center PRESTA study, conducted in Europe, Latin America, and the Asia Pacific region, featured 752 outpatients. During the first phase, patients were randomized to receive etanercept 50 mg once or twice weekly (by subcutaneous injection) for 12 weeks. During the second phase, all subjects received the drug once weekly for 12 more weeks.
At 12 weeks, based on physician’s global assessment, lesions had cleared or almost cleared in 46% of subjects in the twice-weekly group compared to only 32% in the once-weekly group (p < 0.001).
Twice weekly etanercept patients also showed greater mean reductions in the psoriasis area and severity index at week 12 than did once weekly etanercept patients (71% vs. 62%, p < 0.001). By week 24, however, the difference was no longer statistically significant (78% vs. 74%).
In terms of patients achieving arthritis response criteria, by contrast, once weekly etanercept performed just as well as twice weekly (76% vs. 77%). Likewise, joint and tendon manifestations improved to a similar extent in both groups.
Side effects with etanercept were consistent with previous reports on the drug. The most common adverse events were upper respiratory tract infection, injection site reaction, pharyngitis, and headache. There was no significant difference in side effects between groups.
The take-home message, Dr. Sterry said, is that the “skin and joints of patients suffering from psoriasis respond excellently to etanercept treatment, with skin involvement requiring higher dosing” than joint symptoms.
“It might be interesting to see whether other treatments also show this differential effect, and what could be the mechanisms behind it,” he added.
The study was sponsored by Wyeth Pharmaceuticals, which markets etanercept as Enbrel and employs five of the nine authors.
Reference:
BMJ 2010.