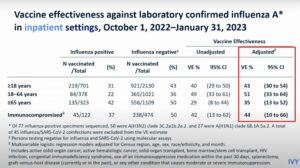
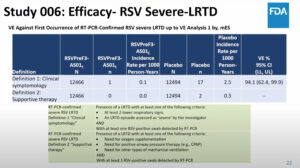
NEW YORK (Reuters Health) – Adding oral methylprednisolone to antibiotic therapy has benefits in children with chronic rhinosinusitis (CRS), according to results of a randomized, double-blind, placebo-controlled study published online May 30 in the Journal of Allergy and Clinical Immunology.
“The place of systemic corticosteroids in the treatment of children with CRS remains unclear,” the study team notes. “There are no previously published placebo-controlled data showing the efficacy of oral corticosteroids in patients with CRS without nasal polyposis.”
A total of 48 children, 6 to 17 years old, with clinically and radiographically proven CRS without nasal polyposis participated in the study. Before entry, they had multiple courses of antimicrobial treatment with at least 2 or more broad-spectrum antibiotics including amoxicillin-clavulanic acid, second generation cephalosporins, or clarithromycin.
At entry, subjects were assigned to oral amoxicillin/clavulanate (AMX/C; 45/6.4 mg/kg/day for 30 days) plus either oral methylprednisolone for 15 days in a tapered schedule (1 mg/kg/day for 10 days, 0.75 mg/kg/day for 2 days, 0.5 mg/kg/day for 2 days, and 0.25 mg/kg/day for 1 day) or matching placebo.
Forty-five subjects completed the trial (22 in the methylprednisolone arm and 23 in the placebo arm). Both groups experienced significant improvements in symptom scores and sinus computed tomographic (CT) scores, the two primary parameters, when comparing baseline values to end-of-treatment values (P < 0.001 for both).
However, methylprednisolone proved significantly more effective than placebo in reducing CT scores (P = 0.004), total rhinosinusitis symptoms (P = 0.001), and individual symptom of nasal obstruction (P = 0.001), postnasal discharge (P = 0.007), and cough (P = 0.009).
At the end treatment, only 14% of children in the methylprednisolone group still had abnormal CT findings compared with 48% of children in the placebo group.
The findings also hint that methylprednisolone might reduce the probability of recurrence in the medium term; there was a trend (P = 0.137) toward fewer clinical relapses in the methylprednisolone arm (25%) compared with the placebo group (43%).
Oral methylprednisolone as an adjunct to standard antibiotic treatment was well tolerated, with no between-group differences in treatment-related adverse events.
An increase in appetite and weight gain was the only reported adverse event and did occur more often in the methylprednisolone arm (73%) than the placebo arm (48%), the authors note. However, average changes from baseline in the weights of patients were not statistically different between groups.
These results, the investigators conclude, suggest that oral methylprednisolone might be a worthwhile adjunct to antibiotic therapy in children and adolescents with CRS.
J Allergy Clin Immunol 2011.