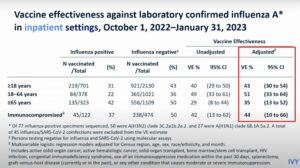
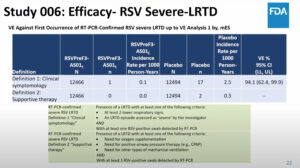
NEW YORK (Reuters Health) – Prophylactic use of acetaminophen during vaccination in children can reduce febrile reactions, but unfortunately the antibody responses to various vaccine antigens are weakened, according to a report in the October 17th issue of The Lancet.
In light of this finding, Dr. Roman Prymula and co-researchers conclude that “prophylactic administration of antipyretic drugs at the time of vaccination should not be routinely recommended.”
Prophylactic antipyretic agents are sometimes given out of concerns for high fever and febrile convulsion, but the efficacy of this intervention and the impact on the vaccine-induced immune response were unclear, Dr. Prymula, from the University of Defence, Hradec Kralove, Czech Republic, and colleagues note.
The current investigation featured two consecutive (primary and booster) open-label randomized, controlled trials. A total of 459 healthy infants were randomized to receive or not receive acetaminophen after immunization with PHiD-CV, DTPa-HBV-IPV/Hib, and oral human rotavirus vaccines.
In both the primary and booster series, the rate of fever over 39.5 degrees Celsius never exceeded 2% in either patient group.
By contrast, the acetaminophen group was less likely than the control group to have temperatures over 38 degrees Celsius. The corresponding rates after primary and booster vaccination were 42% vs. 66% and 36% vs. 58%.
As noted, acetaminophen use was tied to a reduced antibody response. After primary vaccination, lower antibody responses were seen for all ten pneumococcal vaccine serotypes, protein D, antipolyribosyl ribitol phosphate, antidiphtheria, antitetanus, and antipertactin. After booster vaccination, lower responses were observed for antitetanus, protein D, and all pneumococcal serotypes except 19F.
The researchers “present a compelling case against routine use of paracetamol during pediatric immunizations,” Dr. Robert T. Chen, from the Centers for Disease Control and Prevention, Atlanta, and colleagues note in a related editorial. “Whether this rule applies to all antipyretics, all age groups, whole-cell pertussis vaccines that continue to be received by about 70% of the global birth cohort, and subgroups with different risk-benefit considerations requires further study.”
Reference:
Lancet 2009;374:1305-1306,1339-1350.