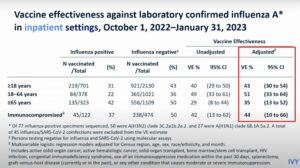
NEW YORK (Reuters Health) – During the 2007-2008 flu season, the licensed inactivated flu vaccine was 50% more effective than the live attenuated vaccine in preventing laboratory-confirmed symptomatic influenza A (predominately H3N2) in healthy adults.
The study included 1952 adults who received either vaccine in the fall of 2007, with H3N2 influenza activity (about 90% type A and 9% type B) documented from January through April 2008.
According to Dr. Arnold S. Monto from University of Michigan School of Public Health, Ann Arbor and colleagues, absolute efficacy against both influenza types — based on culture, real-time PCR, or both — was 68% for the inactivated vaccine compared with 36% for the live attenuated vaccine.
In terms of relative efficacy, there was a 50% reduction in laboratory-confirmed influenza among subjects who received inactivated vaccine versus those who received the live attenuated vaccine, they report in the September 24 issue of the New England Journal of Medicine.
By virus type, the absolute efficacy against influenza type A was 72% with the inactivated vaccine and 29% for the live attenuated vaccine, with a relative efficacy of 60% for the inactivated vaccine.
These data